
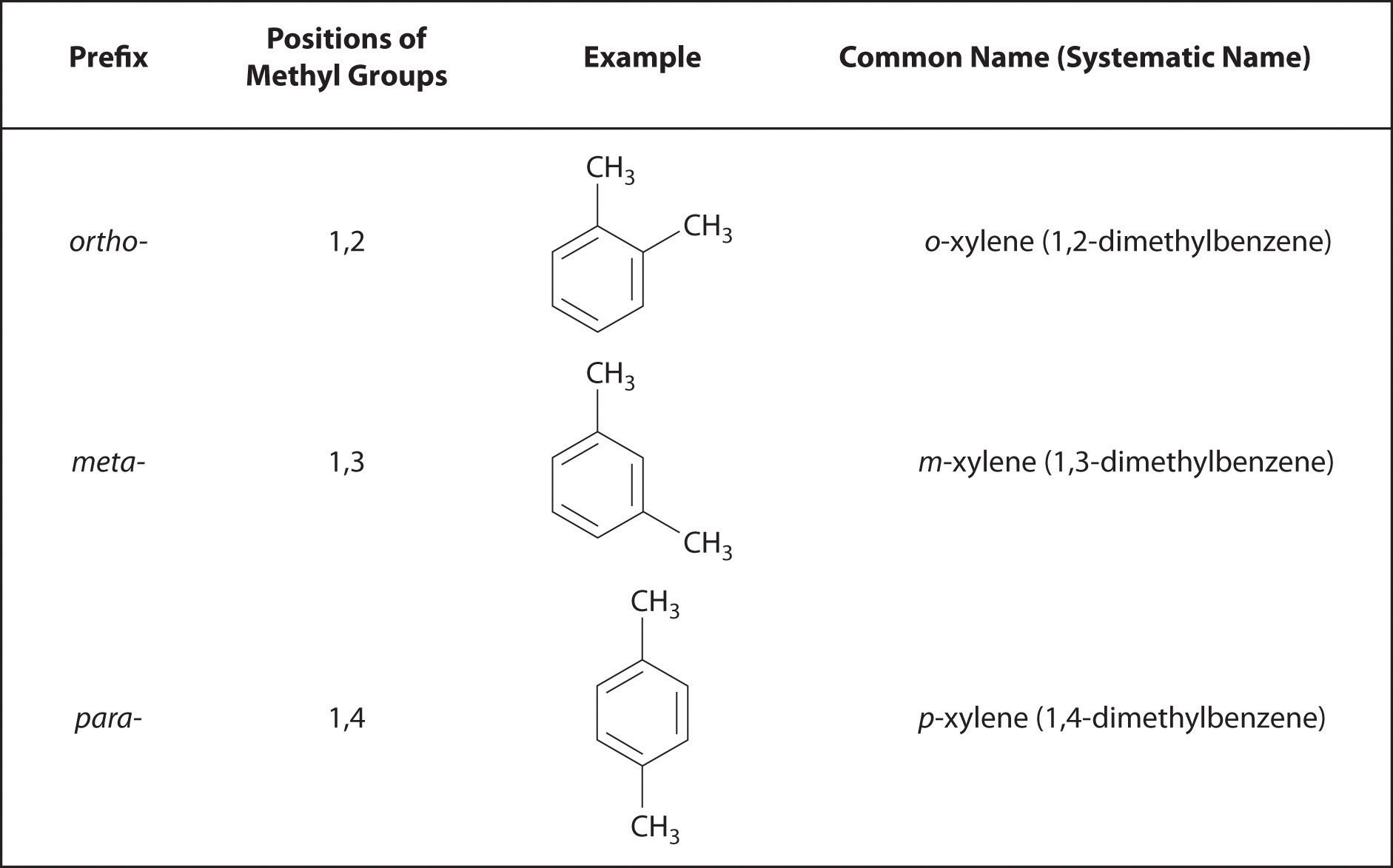
But the above list will give you what you need to continue on.Īnd don't just look at the cards. Every functional group below is eventually discussed at one point or another in the book. After you've learned all these, add a couple more cards and learn those.
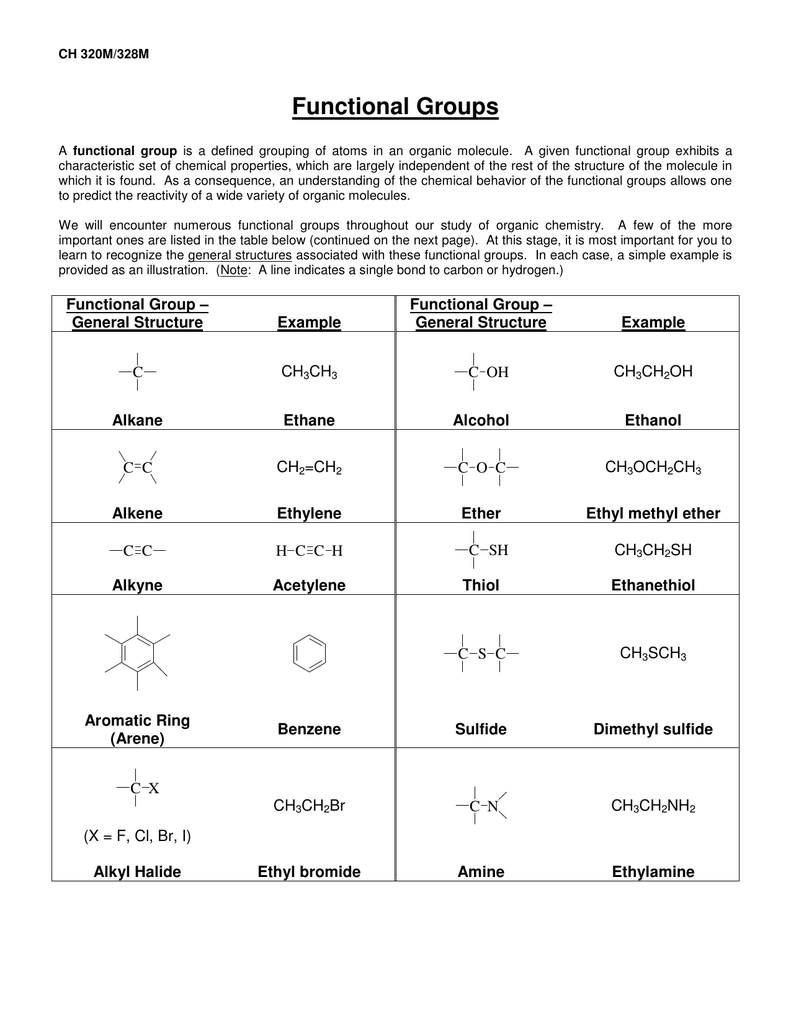
Your initial set of cards should include, at the very least: Alkene, Alkyne, Alkyl halide (or Haloalkane), Alcohol, Aldehyde, Ketone, Carboxylic Acid, Acyl Chloride (or Acid Chloride), Ester, Ether, Amine, Sulfide, and Thiol. Get a pack of index cards and write the name of the functional group on one side, and draw its chemical representation on the other.įor now, a list of the most important ones you should know is provided here. One of the easiest ways to learn functional groups is by making flash cards. It's like trying to learn French without first learning the meaning of some of the words. It's simply impossible to discuss chemistry without knowing the "lingo". It will be assumed that the student is familiar with most of the ones in the tables below. As you proceed through the text, the writing will be in terms of functional groups. Memorizing Functional Groups ĭon't assume that you can simply skim over the functional groups and move on. Organic reactions usually take place at the functional group, so learning about the reactivities of functional groups will prepare you to understand many other things about organic chemistry. An -OH group on one molecule will tend to react similarly, although perhaps not identically, to an -OH on another molecule. Just as elements have distinctive properties, functional groups have characteristic chemistries. It could be found on any number of different molecules. A functional group makes up part of a larger molecule.įor example, -OH, the hydroxyl group that characterizes alcohols, is an oxygen with a hydrogen attached. The identification of functional groups and the ability to predict reactivity based on functional group properties is one of the cornerstones of organic chemistry.įunctional groups are specific atoms, ions, or groups of atoms having consistent properties. These parts of organic molecules are called functional groups. Fortunately, organic chemicals consist of a relatively few similar parts, combined in different ways, that allow us to predict how a compound we have never seen before may react, by comparing how other molecules containing the same types of parts are known to react. In fact, there are many times more organic compounds known than all the other (inorganic) compounds discovered so far, about 7 million organic compounds in total. The number of known organic compounds is quite large.


 0 kommentar(er)
0 kommentar(er)
